By Prof. L. Kaliambos (Natural Philosopher in New Energy)
March 11, 2023
Today it is well-known that the Nobel prize in physics (2022) confirmed Newton's third law of instantaneous interaction (faster than light) and rejected Maxwell's and Einstein's invalid fields, as I presented them at an international conference on physics in 1993 and at a nuclear conference at NCSR "Democritos" in 2002. (2022 NOBEL PRIZE WINNERS proved Einstein WRONG). Historically in 1925 the two young Dutch physicists Uhlenbeck and Goudsmit discovered the electron spin according to which the peripheral velocity of a spinning electron is faster than the speed of light. Since this discovery invalidates Einstein’s relativity it met much opposition by physicists including Pauli. Nevertheless surprisingly in my paper "Nuclear structure is governed by the fundamental laws of electromagnetism" (2003) I showed that the peripheral velocity (u) of the electron spin is greater than light ( u >> c ), which explains all atomic and molecular phenomena. Therefore I published my paper "SPIN-SPIN INTERACTIONS OF ELECTRONS AND ALSO OF NUCLEONS CREATE ATOMIC MOLECULAR AND NUCLEAR STRUCTURES (2008) for finding the ground state energies of all atoms. For example I have found a closed- form solution to the Schrodinger equation for the helium atom, based on the stronger magnetic attraction than the electric repulsion of two electrons of opposite spin at very small distances. Whereas in the dominant article "HELIUM ATOM-WIKIPEDIA" under the ACADEMIC ESTABLISHMENT OF WRONG THEORIES we read incorrectly the following various approximations: "Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found. However, various approximations, such as the Hartree–Fock method, can be used to estimate the ground state energy and wavefunction of the atom."
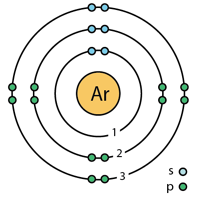
Argon is a chemical element with symbol Ar and atomic number 18. Unlike for hydrogen of one electron, a closed-form solution to the Schrödinger equation for the many-electron atoms like the Argon atom has not been found. So, under the invalid relativity (EXPERIMENTS REJECT RELATIVITY) various approximations, such as the Hartree–Fock method, could be used to estimate the ground state energies. Under these difficulties I published my paper “ Spin-spin interactions of electrons and also of nucleons create atomic molecular and nuclear structures" (2008) by nalyzing carefully the electromagnetic interactions of two spinning electrons of opposite spin which give a simple formula for the solution of such ground state energies. Hence for Argon we may use this correct image with the following electron configuration:
1s2.2s2.2px2.2py2.2pz2.3s2.3px2.3py2 .3pz2 .
According to the “Ionization energies of the elements-WIKIPEDIA” the ionization energies (in eV) are the following: E1 = 15.76 , E2 = 27.63 , E3 = 40.74 , E4 = 59.81 , E5 = 75, E6 = 91, E7 = 124.32, E8 = 143.46, E9 = 422.45 , E10 = 478.7 , E11 = 538.96 , E12 = 618.26. E13 = 686.1, E14 = 755.74, E15 = 854.77 , E16 = 918 , E17 = 4120.89 and E18 = 4426.23
Here the -( E1 + E2 + E3 + E4 + E5 + E6 ) equals the binding energy E( 3px2 + 3py2 + 3pz2) of the 6 outer electrons. Then the -( E7 + E8) equals the binding energy E(3s2). Also, the -( E9 + E10 + E11 + E12 + E13 + E14 ) equals the binding energy E( 2px2 + 2py2 + 2pz2) . On the other hand the -( E15 + E16 ) equals the binding energy E(2s2) , while the -( E17 + E18) equals the binding energy E(1s2). See also my papers about the explanation of ionization energies of elements in my FUNDAMENTAL PHYSICS CONCEPTS. .
EXPLANATION OF ( E1 + E2 + E3 + E4 + E5 + E6 ) = 309.94 eV = -E(3px2 + 3py2 + 3pz2)
Here the E(3px2 + 3py2 + 3pz2 ) represents the binding energy of the 6 outer paired electrons given by applying my formula of 2008. The charges (-12e) of the electrons 1s2.2s2.2px2.2py2.2pz2.3ps2 screen the nuclear charge (+18e) and for a perfect screening we would have an effective Zeff = ζ = 6. However the 6 outer electrons (3px2 + 3py2 + 3pz2 repel the 3ps2 electrons and lead to the deformation of shells with ζ > 6. Under this condition we may write
(E1 +… + E6) = - 3[(-27.21)ζ2 +(16.95)ζ - 4.1]/n2
Since n = 3 and ( E1 + Ε2 + E3 + E4 + E5 + E6 ) = 309.94 eV the above equation can be written as
9.07ζ2 - 5.65ζ - 308.57 = 0
Then, solving for ζ we get ζ = 6.15 > 6
Here 6.15 > 6 means that the 6 outer electrons ( 3px2 + 3py2 + 3pz2) make a spherical shell leading almost to perfect screening
EXPLANATION OF ( E7 + E8 ) = 267.78 eV = -E(3s2)
Here the E(3s2) represents the binding energy of the two electrons (3s2) given by applying my formula of 2008. The charges (-10e) of the electrons of 1s2.2s2.2px2.2py2.2pz2 screen the nuclear charge (+18e) and for a perfect screening we would have an effective ζ = 8. However the two electrons of 3s2 penetrate the 2px2.2py2.2pz2 leading to the deformation of shells with ζ > 8. Under this condition we write the following equation as
( E7 + E8 ) = 267.78 eV = - E(3s2) = - [(- 27.21)ζ2 + ( 16.95) ζ - 4.1 ]/n2
Since n = 3 we may write
3.0233ζ2 - 1.8833ζ - 267.3244 = 0
Then solving for ζ we get ζ = 9.72 > 8
EXPLANATION OF -(E9 + E10 + E11 + E12 + E13 + E14 ) = - 3500.2 = E(2px2 + 2py2 + 2pz2)
Here E(2px2 + 2py2 + 2pz2) represent the binding energy of the six paired electrons. The charges (-4e) of the four electrons 1s2.2s2 screen the nuclear charge (+18e) and for a perfect screening we would have ζ = 14, because +18e - 4e = + 14e. Thus for n = 2 we should determine the effective ζ = 14 or ζ > 14 of the total binding energy of 6 paired electrons by applying my formula of 2008 as
E (2px2 + 2py2 + 2pz2) = 3[(-27.21)ζ2 + (16.95)ζ - 4.1)] / n2 = - 3500.2
Surprisingly solving for ζ we get ζ < 14 , which cannot exist. In fact, since 2px2 , 2py2 , and 2pz2 make a complete spherical shell they provide a perfect screening with ζ = 14. So using ζ = 14 and solving for n we expect to find n > 2, because a perfect screening after the experiments of ionizations means that the quantum number n = 2 becomes n > 2. Under this condition for determining here the quantum number n the above equation could be written as
E(2px2 + 2py2 + 2pz2) = 3[(-27.21)142 + (16.95)14 - 4.1 )]/n2 = - 3500.2
Then solving for n we get n = 2.09 > 2
In other words the three orbitals of paired electrons do not lead to the deformations of 1s2 and 2s2 but differ from the symmetry of (2px1+ 2py1 +2pz1) which exert both electric and magnetic repulsions. Here the electric repulsions between the paired electrons of 2px2, 2py2 and 2pz2 make a complete spherical shell and lead to a perfect screening with ζ = 14. Under this condition the quantum number n = 2 becomes n = 2.09
Note that the two electrons of opposite spin (say the 2px2) do not provide any mutual repulsion because I discovered in 2008 that at very short inter-electron separations the magnetic attraction is stronger than the electric repulsion giving a vibration energy.
However in the absence of a detailed knowledge about the mutual electromagnetic interaction between the electrons of the 2px2 or 2py2 or 2pz2 , today many physicists believe incorrectly that it is due to the Coulomb repulsion between the two electrons of opposite spin. Under such fallacious ideas I published my paper of 2008.
EXPLANATION OF ( E15 + E16 ) = 1772.8 eV = - E (2s2)
Here the E(2s2) represents the binding energy of the two paired electrons ( 2s2). The charges (-2e) of 1s2 screen the nuclear charge (+18e) and for a perfect screening we would have an effective ζ = 16. However according to the quantum mechanics the two electrons (2s2) penetrate the 1s2 shell. Thus they lead to the deformations of both 1s2 and 2s2 spherical shells giving an effective ζ > 16. Since n = 2 we apply my formula of 2008 to write
( E15 + E16 ) = - E(2s2) = - [(-27.21) )ζ2 + (16.95) ζ - 4.1 ] / 22
Since ( E15 + E16 ) = 1772.8 eV, we may rewrite
6.8025ζ2 - 4.2375ζ - 1771.775 = 0
Then, solving for ζ we get ζ = 16.45 > 16 .
Here ζ = 16.45 > 16 means that the repulsιοns (2s2-1s2 ) lead to the deformation of shells, because the two electrons (2s2) or (1s2) of opposite spin behave like one particle. Note that in both cases the repulsions are due to only electric forces of the Coulomb law. Whereas in the case of the three electrons of 3px1, 3py1, and 3pz1 of parallel spin (S = 1) the three electrons interact with both electric and magnetic repulsions from symmetrical positions.
EXPLANATION OF -( Ε17 + E18 ) = - 8547.12 = E( 1s2)
As in the case of helium the binding energy E(1s2) is due to the two remaining electrons of 1s2 with n = 1. Thus we may calculate the binding energy by applying my formula of 2008 for Z = 18 as
E(1s2) = [(-27.21) )182 + (16.95)18 - 4.1 ] /12 = - 8515.04
However the experiments of ionizations give - (E17 + E18 ) = - 8547.12 . In other words one sees here that after the ionizations my formula of 2008 gives the value of 8515.04 eV which is smaller than the experimental value of 8547.12 eV. Under this condition of ionizations I suggest that n = 1 becomes n < 1 due to the fact that the ionizations reduce the electron charges and now the nuclear charge is much greater than the electron charge of the two remaining electrons. So for Z =18 we determine the n by writing
(E17 + E18 ) = 8547.12 eV = - E(1s2) = - [(-27.21) )182 + (16.95)18 - 4.1 ] /n2
Then solving for n we get n = 0.998.