By Prof. L. Kaliambos (Natural Philosopher in New Energy)
January 24, 2016
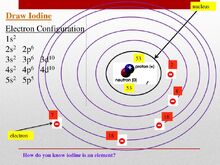
Iodine is an atom of the chemical element iodine with symbol I and atomic number 53. However unlike for hydrogen atom, a closed-form solution to the Schrödinger equation for the many-electron atoms like the iodine atom has not been found. So, various approximations, such as the Hartree–Fock method, could be used to estimate the ground state energies. Under these difficulties I analyzed carefully the electromagnetic interactions of two spinning electrons of opposite spin which give a simple formula for the solution of such ground state energies. You can see it in my published paper “ Spin-spin interactions of electrons and also of nucleons create atomic molecular and nuclear structures”.after my published paper "Spin-spin interactions of electrons and also of nucleons create atomic molecular end nuclear structures" (2008). Under this condition today it is well known that the correct electron configuration should be given by this image of Iodine including the following electron configuration:
1s2.2s2.2px2.2py2.2pz2.3s2.3px2.3py2.3pz2.3d10. 4s2. 4p6.4d10. 5s2.5px25py25pz1
According to the “Ionization energies of the elements-WIKIPEDIA” the ionization energies (eV) of iodine (from (E1 to E3 ) are the following:
E1 = 10.45 , E2 = 19.13, and E3 = 33 .
Here the E1 represents the ionization energy of the one electron of 5px2 . Also the E2 represents the ionization energy of the one electron of 5py2 . Therefore the E3 represents the ground state energy of 5px1 Note that in the absence of more ionization energies we cannot calculate the ground state energies of inner orbitals. Nevertheless for understanding better such ground state energies you can see also my papers about the ground stae energies of elements in my FUNDAMENTAL PHYSICS CONCEPTS. Moreover in “User : Kaliambos” you can see my paper of 2008.
GROUND STATE ENERGY OF -E3 = -33 eV = E(5x1)
Here the E(5px1) represents the ground state energy of the 5px1.The electron charges (-48e) of (1s22s22p63s23p63d104s2 4p64d10 5s2) screen the nuclear charge (+53e) and for a perfect screening we would have the same ζ = 5. However the electrons of 5p break more the symmetry and lead to the deformations of electron clouds. Thus ζ > 5.
Since 5px1 is the one electron of parallel spin we apply the Bohr formula to write
E3 = -E(5px1) = 33 eV = - (-13.6057)ζ2/n2
Therefore E3 = 33 eV = (13.6057)ζ2 / 52
Then we get ζ = 7.79 > 5 .
Here 7.79 > 7.66 > 5 means that after the ionizations the electrons of 5p break more the symmetry and lead to a great deformation of electron clouds.
WHY EINSTEIN’S WRONG RELATIVITY LED TO THE ABANDONMENT OF NATURAL LAWS IN FAVOR OF WRONG ATOMIC THEORIES
It is of interest to note that in the absence of a detailed knowledge about the electromagnetic interaction of two electrons of opposite spin physicists so far under the influence of Einstein’s relativity could not calculate such ground state energies of many-electron atoms. Historically, despite the enormous success of the Bohr model and the quantum mechanics of the Schrodinger equation based on the well-established laws of electromagnetism in explaining the principal features of the hydrogen spectrum and of other one-electron atomic systems, so far, under the influence of Einstein’s relativity which led to the abandonment of natural laws neither was able to provide a satisfactory explanation of the two-electron atoms.
In atomic physics a two-electron atom is a quantum mechanical system consisting of one nucleus with a charge Ze and just two electrons. This is the first case of many-electron systems. The first few two-electron atoms are:
Z =1 : H- hydrogen anion. Z = 2 : He helium atom. Z = 3 : Li+ lithium atom anion. Z = 4 : Be2+ beryllium ion.
Prior to the development of quantum mechanics, an atom with many electrons was portrayed like the solar system, with the electrons representing the planets circulating about the nuclear “sun”. In the solar system, the gravitational interaction between planets is quite small compared with that between any planet and the very massive sun; interplanetary interactions can, therefore, be treated as small perturbations.
However, In the helium atom with two electrons, the interaction energy between the two spinning electrons and between an electron and the nucleus are almost of the same magnitude, and a perturbation approach is inapplicable.
In 1925 the two young Dutch physicists Uhlenbeck and Goudsmit discovered the electron spin according to which the peripheral velocity of a spinning electron is greater than the speed of light. Since this discovery invalidates Einstein’s relativity it met much opposition by physicists including Pauli. Under the influence of Einstein’s invalid relativity (EXPERIMENTS REJECTING EINSTEIN) physicists believed that in nature cannot exist velocities faster than the speed of light.(See my FASTER THAN LIGHT).
So, great physicists like Pauli, Heisenberg, and Dirac abandoned the natural laws of electromagnetism in favor of wrong theories including qualitative approaches under an idea of symmetry properties between the two electrons of opposite spin which lead to many complications. Thus, in the “Helium atom-Wikipedia” one reads: “Unlike for hydrogen a closed form solution to the Schrodinger equation for the helium atom has not been found. However various approximations such as the Hartree-Fock method ,can be used to estimate the ground state energy and wave function of atoms”.
It is of interest to note that in 1993 in Olympia of Greece I presented at the international conference “Frontiers of fundamental physics” my paper of dipole photons. The conference was organized by the natural philosophers M. Barone and F. Selleri, who awarded me an award including a disc of the atomic philosopher Democritus because in that paper I showed that LAWS AND EXPERIMENTS INVALIDATE FIELDS AND RELATIVITY . At the same period I tried to find not only the nuclear force and structure but also the coupling of two electrons under the application of the abandoned electromagnetic laws. For example in the photoelectric effect the absorption of light contributed not only to the increase of the electron energy but also to the increase of the electron mass, because the particles of light have mass m = hν/c2. ( See my paper "DISCOVERY OF PHOTON MASS" ).
However the electron spin which gives a peripheral velocity greater than the speed of light cannot be affected by the photon absorption. Thus after 10 years I published my paper "Nuclear structure ..electromagnetism" (2003), in which I showed not only my DISCOVERY OF NUCLEAR FORCE AND STRUCTURE but also that the peripheral velocity (u >> c) of two spinning electrons with opposite spin gives an attractive magnetic force (Fm) stronger than the electric repulsion (Fe) when the two electrons of mass m and charge (-e) are at a very short separation (r < 578.8 /1015 m). Because of the antiparallel spin along the radial direction the interaction of the electron charges gives an electromagnetic force
Fem = Fe - Fm .
Therefore in my research the integration for calculating the mutual Fem led to the following relation:
Fem = Fe - Fm = Ke2/r2 - (Ke2/r4)(9h2/16π2m2c2)
Of course for Fe = Fm one gets the equilibrium separation ro = 3h/4πmc = 578.8/1015 m. That is, for an interelectron separation r < 578.8/1015 m the two electrons of opposite spin exert an attractive electromagnetic force, because the attractive Fm is stronger than the repulsive Fe . Here Fm is a spin-dependent force of short range. As a consequence this situation provides the physical basis for understanding the pairing of two electrons described qualitatively by the Pauli principle, which cannot be applied in the simplest case of the deuteron in nuclear physics, because the binding energy between the two spinning nucleons occurs when the spin is not opposite (S=0) but parallel (S=1). According to the experiments in the case of two electrons with antiparallel spin the presence of a very strong external magnetic field gives parallel spin (S=1) with electric and magnetic repulsions given by
Fem = Fe + Fm
So, according to the well-established laws of electromagnetism after a detailed analysis of paired electrons in two-electron atoms I concluded that at r < 578.8/1015 m a motional EMF produces vibrations of paired electrons.
Unfortunately today many physicists in the absence of a detailed knowledge believe that the two electrons of two-electron atoms under the Coulomb repulsion between the electrons move not together as one particle but as separated particles possessing the two opposite points of the diameter of the orbit around the nucleus. In fact, the two electrons of opposite spin behave like one particle circulating about the nucleus under the rules of quantum mechanics forming two-electron orbitals in helium, beryllium etc. In my paper of 2008, I showed that the positive vibration energy (Ev) described in eV depends on the Ze charge of nucleus as
Ev = 16.95Z - 4.1
Of course in the absence of such a vibration energy Ev it is well-known that the ground state energy E described in eV for two orbiting electrons could be given by the Bohr model as
E = (-27.21) Z2.
So the combination of the energies of the Bohr model and the vibration energies due to the opposite spin of two electrons led to my discovery of the ground state energy of two-electron atoms given by
-E = (-27.21) Z2 + (16.95 )Z - 4.1
For example the laboratory measurement of the ionization energy of H- yields an energy of the ground state -E = - 14.35 eV. In this case since Z = 1 we get
-E = -27.21 + 16.95 - 4.1 = -14.35 eV. In the same way writing for the helium Z = 2 we get
-E = - 108.8 + 32.9 - 4.1 = -79.0 eV
The discovery of this simple formula based on the well-established laws of electromagnetism was the first fundamental equation for understanding the energies of many-electron atoms, while various theories based on qualitative symmetry properties lead to complications.