By Prof. L. Kaliambos (Natural Philosopher in New Energy)
October 27, 2015
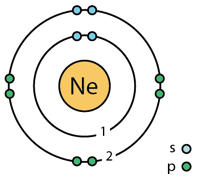
Neon is an atom of the chemical element neon with symbol Ne and atomic number 10. However unlike for hydrogen, a closed-form solution to the Schrödinger equation for the many-electron atoms like the neon atom has not been found. So, various approximations, such as the Hartree–Fock method, could be used to estimate the ground state energies. Under these difficulties I published my paper “ Spin-spin interactions of electrons and also of nucleons create atomic molecular and nuclear structures” (2008) by analyzing carefully the electromagnetic interactions of two spinning electrons of opposite spin. Under this condition the correct electron configuration should be give by this image of Neon including the following electron configuration: 1s2.2s2.2px2.2py2.2pz2.
According to the “Ionization energies of the elements-WIKIPEDIA” the ionization energies (in eV) of the neon atom are the following: E1 = 21.5646 , E2 = 40.96328 , E3 = 63.45 , E4 = 97.12 , E5 = 126.21 , E6 = 157.93, E7 = 207.2759 , E8 = 239.0989 . E9 = 1195.8286 , and E10 = 1362.1995. See also my papers about the explanation of ionization energies of the elements in my FUNDAMENTAL PHYSICS CONCEPTS based on my paper “ Spin-spin interactions of electrons and also of nucleons create atomic molecular and nuclear structures” published in Ind. J. Th. Phys. (2008).
For understanding the ionization energies E1, E2, E3 , E4 , E5 , E6 , E7 and E8 which give the ground state energies of (2s2.2px2.2py2.2pz2) you can see my “EXPLANATION OF NEON IONIZATIONS”.
In the same way for the explanation of the E9 and E10 which give the ground state energy of the 1s2 electrons one must apply both the Bohr model and my formula of my paper of 2008.
EXPLANATION OF Ε10 = 1362.1995 eV AND E9 = 1195.8286 eV, WHICH GIVE THE GROUND STATE ENERGY OF 1s2 ELECTRONS
As in the case of the helium the ionization energy E10 = 1362.1995 eV = - E(1s1) is due to the one remaining electron of 1s1 with n = 1. Thus we expect to calculate it by applying the simple Bohr model for Z = 10 as
E10 = - (-13.6057 )Z2 /12 = - (-13.6057)10 2 /12 = 1360.57 eV.
Surprisingly one sees here that after the ionizations the Bohr model gives the value of 1360.57 eV which is smaller than the experimental value of E10 = 1362.1995. Under this condition of ionizations I suggest that n = 1 becomes n < 1 due to the fact that the ionizations reduce the electron charges and now the nuclear charge is much greater than the electron charge of the remaining electron. So we may write
E10 = 1362.1995 eV = (13.6057) Z2/n2 = (13.6057)102/n2
Then solving for n we get n = 0.9994.
In the same way for calculating the E9 = 1195.8286 eV we must determine the simple quantum number n < 1 by applying my formula of 2008 as
E9 = 1195.8286 eV = - E10 - E(1s2) = -1362.1995 - [(-27.21)102 + (16.95)10 - 4.1 ] / n2
Then solving for n we get n = 0.9995.
Here 0.9995 > 0.9994, because the second electron increase the electron charge with respect to nuclear charge.
However in the absence of a detailed knowledge about the electromagnetic force between the two spinning electrons of opposite spin physicist today using wrong theories cannot explain the ground state energy of the electrons 1s2. For example under wrong theories based on qualitative approaches many physicists believe incorrectly that the second electron of the 1s2 shell is less tightly bound because it could be interpreted as a shielding effect; the other electron partly shields the second electron from the full charge of the nucleus. Another wrong way to view the energy is to say that the repulsion of the electrons contributes a positive potential energy which partially offsets the negative potential energy contributed by the attractive electric force of the nuclear charge.
Under such false ideas I published my paper of 2008 . You can see the paper in “User Kaliambos”.
Historically, despite the enormous success of the Bohr model and the quantum mechanics of the Schrodinger equation based on the well-established laws of electromagnetism in explaining the principal features of the hydrogen spectrum and of other one-electron atomic systems, so far, under the abandonment of natural laws neither was able to provide a satisfactory explanation of the two-electron atoms. In atomic physics a two-electron atom is a quantum mechanical system consisting of one nucleus with a charge Ze and just two electrons. This is the first case of many-electron systems. The first few two-electron atoms are:
Z =1 : H- hydrogen anion. Z = 2 : He helium atom. Z = 3 : Li+ lithium atom anion. Z = 4 : Be2+ beryllium ion.
Prior to the development of quantum mechanics, an atom with many electrons was portrayed like the solar system, with the electrons representing the planets circulating about the nuclear “sun”. In the solar system, the gravitational interaction between planets is quite small compared with that between any planet and the very massive sun; interplanetary interactions can, therefore, be treated as small perturbations.
However, In the helium atom with two electrons, the interaction energy between the two spinning electrons and between an electron and the nucleus are almost of the same magnitude, and a perturbation approach is inapplicable.
In 1925 the two young Dutch physicists Uhlenbeck and Goudsmit discovered the electron spin according to which the peripheral velocity of a spinning electron is greater than the speed of light. Since this discovery invalidates Einstein’s relativity it met much opposition by physicists including Pauli. Under the influence of Einstein’s invalid relativity physicists believed that in nature cannot exist velocities faster than the speed of light.(See my FASTER THAN LIGHT).
So, great physicists like Pauli, Heisenberg, and Dirac abandoned the natural laws of electromagnetism in favor of wrong theories including qualitative approaches under an idea of symmetry properties between the two electrons of opposite spin which lead to many complications. Thus, in the “Helium atom-Wikipedia” one reads: “Unlike for hydrogen a closed form solution to the Schrodinger equation for the helium atom has not been found. However various approximations such as the Hartree-Fock method ,can be used to estimate the ground state energy and wave function of atoms”.
It is of interest to note that in 1993 in Olympia of Greece I presented at the international conference “Frontiers of fundamental physics” my paper “Impact of Maxwell’s equation of displacement current on electromagnetic laws and comparison of the Maxwellian waves with our model of dipolic particles ". The conference was organized by the natural philosophers M. Barone and F.Selleri , who awarded me an award including a disc of the atomic philosopher Democritus, since in that paper I showed that LAWS AND EXPERIMENTS INVALIDATE FIELDS AND RELATIVITY . At the same period I tried to find not only the nuclear force and structure but also the coupling of two electrons under the application of the abandoned electromagnetic laws. For example in the photoelectric effect the absorption of light contributed not only to the increase of the electron energy but also to the increase of the electron mass, because the particles of light have mass m = hν/c2. ( See my paper "DISCOVERY OF PHOTON MASS" ).
However the electron spin which gives a peripheral velocity greater than the speed of light cannot be affected by the photon absorption. Thus after 10 years I published my paper "Nuclear structure..electromagnetism" (2003), in which I showed not only my DISCOVERY OF NUCLEAR FORCE AND STRUCTURE but also that the peripheral velocity (u >> c) of two spinning electrons with opposite spin gives an attractive magnetic force (Fm) stronger than the electric repulsion (Fe) when the two electrons of mass m and charge (-e) are at a very short separation (r < 578.8 /1015 m). Because of the antiparallel spin along the radial direction the interaction of the electron charges gives an electromagnetic force
Fem = Fe - Fm .
Therefore in my research the integration for calculating the mutual Fem led to the following relation:
Fem = Fe - Fm = Ke2/r2 - (Ke2/r4)(9h2/16π2m2c2)
Of course for Fe = Fm one gets the equilibrium separation ro = 3h/4πmc = 578.8/1015 m.
That is, for an interelectron separation r < 578.8/1015 m the two electrons of opposite spin exert an attractive electromagnetic force, because the attractive Fm is stronger than the repulsive Fe . Here Fm is a spin-dependent force of short range. As a consequence this situation provides the physical basis for understanding the pairing of two electrons described qualitatively by the Pauli principle, which cannot be applied in the simplest case of the deuteron in nuclear physics, because the binding energy between the two spinning nucleons occurs when the spin is not opposite (S=0) but parallel (S=1). According to the experiments in the case of two electrons with antiparallel spin the presence of a very strong external magnetic field gives parallel spin (S=1) with electric and magnetic repulsions given by
Fem = Fe + Fm
So, according to the well-established laws of electromagnetism after a detailed analysis of paired electrons in two-electron atoms I concluded that at r < 578.8/1015 m a motional EMF produces vibrations of paired electrons.
Unfortunately today many physicists in the absence of a detailed knowledge believe that the two electrons of two-electron atoms under the Coulomb repulsion between the electrons move not together as one particle but as separated particles possessing the two opposite points of the diameter of the orbit around the nucleus. In fact, the two electrons of opposite spin behave like one particle circulating about the nucleus under the rules of quantum mechanics forming two-electron orbitals in helium, beryllium etc. In my paper of 2008, I showed that the positive vibration energy (Ev) described in eV depends on the Ze charge of nucleus as
Ev = 16.95Z - 4.1
Of course in the absence of such a vibration energy Ev it is well-known that the ground state energy E described in eV for two orbiting electrons could be given by the Bohr model as
E = (-27.21) Z2.
So the combination of the energies of the Bohr model and the vibration energies due to the opposite spin of two electrons led to my discovery of the ground state energy of two-electron atoms given by
-E = (-27.21) Z2 + (16.95 )Z - 4.1
For example the laboratory measurement of the ionization energy of H- yields an energy of the ground state -E = - 14.35 eV. In this case since Z = 1 we get
-E = -27.21 + 16.95 - 4.1 = -14.35 eV. In the same way writing for the helium Z = 2 we get
-E = - 108.8 + 32.9 - 4.1 = -79.0 eV
The discovery of this simple formula based on the well-established laws of electromagnetism was the first fundamental equation for understanding the energies of many-electron atoms, while various theories based on qualitative symmetry properties lead to complications.